| dc.contributor | Vall d'Hebron Barcelona Hospital Campus |
| dc.contributor.author | Quintela-Fandino, Miguel |
| dc.contributor.author | Holgado, Esther |
| dc.contributor.author | Manso, Luis |
| dc.contributor.author | Bermejo, Begoña |
| dc.contributor.author | Colomer, Ramon |
| dc.contributor.author | Cortés Castan, Javier |
| dc.contributor.author | Morales, Serafin |
| dc.date.accessioned | 2021-09-10T10:07:06Z |
| dc.date.available | 2021-09-10T10:07:06Z |
| dc.date.issued | 2020-11-11 |
| dc.identifier.citation | Quintela-Fandino M, Holgado E, Manso L, Morales S, Bermejo B, Colomer R, et al. Immuno-priming durvalumab with bevacizumab in HER2-negative advanced breast cancer: a pilot clinical trial. Breast Cancer Res. 2020 Nov 11;22:124. |
| dc.identifier.issn | 1465-542X |
| dc.identifier.uri | https://hdl.handle.net/11351/6299 |
| dc.description | Bevacizumab; Durvalumab; HER2-negative breast cancer |
| dc.description.abstract | Background
Preclinical research suggests that the efficacy of immune checkpoint inhibitors in breast cancer can be enhanced by combining them with antiangiogenics, particularly in a sequential fashion. We sought to explore the efficacy and biomarkers of combining the anti-PD-L1 durvalumab plus the antiangiogenic bevacizumab after bevacizumab monotherapy for advanced HER2-negative breast cancer.
Methods
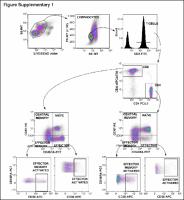
Patients had advanced HER2-negative disease that progressed while receiving single-agent bevacizumab maintenance as a part of a previous chemotherapy plus bevacizumab regimen. Treatment consisted of bi-weekly durvalumab plus bevacizumab (10 mg/kg each i.v.). Peripheral-blood mononuclear cells (PBMCs) were obtained before the first durvalumab dose and every 4 weeks and immunophenotyped by flow-cytometry. A fresh pre-durvalumab tumor biopsy was obtained; gene-expression studies and immunohistochemical staining to assess vascular normalization and characterize the immune infiltrate were conducted. Patients were classified as “non-progressors” if they had clinical benefit (SD/PR/CR) at 4 months. The co-primary endpoints were the changes in the percentage T cell subpopulations in PBMCs in progressors versus non-progressors, and PFS/OS time.
Results
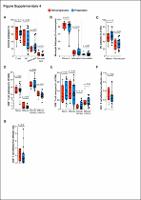
Twenty-six patients were accrued. Median PFS and OS were 3.5 and 11 months; a trend for a longer OS was detected for the hormone-positive subset (19.8 versus 7.4 months in triple-negatives; P = 0.11). Clinical benefit rate at 2 and 4 months was 60% and 44%, respectively, without significant differences between hormone-positive and triple-negative (P = 0.73). Non-progressors’ tumors displayed vascular normalization features as a result of previous bevacizumab, compared with generally abnormal patterns observed in progressors. Non-progressors also showed increased T-effector and T-memory signatures and decreased TREG signatures in gene expression studies in baseline—post-bevacizumab—tumors compared with progressors. Notably, analysis of PBMC populations before durvalumab treatment was concordant with the findings in tumor samples and showed a decreased percentage of circulating TREGs in non-progressors.
Conclusions
This study reporting on sequential bevacizumab+durvalumab in breast cancer showed encouraging activity in a heavily pre-treated cohort. The correlative studies agree with the preclinical rationale supporting an immunopriming effect exerted by antiangiogenic treatment, probably by reducing TREGs cells both systemically and in tumor tissue. The magnitude of this benefit should be addressed in a randomized setting. |
| dc.language.iso | eng |
| dc.publisher | BMC |
| dc.relation.ispartofseries | Breast Cancer Research;22 |
| dc.rights | Attribution 4.0 International |
| dc.rights.uri | http://creativecommons.org/licenses/by/4.0/ |
| dc.source | Scientia |
| dc.subject | Mama - Càncer |
| dc.subject | Medicaments antineoplàstics - Ús terapèutic |
| dc.subject.mesh | Breast Neoplasms |
| dc.subject.mesh | Antineoplastic Combined Chemotherapy Protocols |
| dc.title | Immuno-priming durvalumab with bevacizumab in HER2-negative advanced breast cancer: a pilot clinical trial |
| dc.type | info:eu-repo/semantics/article |
| dc.identifier.doi | 10.1186/s13058-020-01362-y |
| dc.subject.decs | neoplasias de la mama |
| dc.subject.decs | protocolos de quimioterapia antineoplásica combinada |
| dc.relation.publishversion | https://doi.org/10.1186/s13058-020-01362-y |
| dc.type.version | info:eu-repo/semantics/publishedVersion |
| dc.audience | Professionals |
| dc.contributor.organismes | Institut Català de la Salut |
| dc.contributor.authoraffiliation | [Quintela-Fandino M] Breast Cancer Clinical Research Unit – Clinical Research Program, CNIO - Spanish National Cancer Research Center, Melchor Fernandez Almagro, 3, 28029 Madrid, Spain. Medical Oncology Department, Hospital Universitario de Fuenlabrada, Fuenlabrada, Spain. Medical Oncology Department, Hospital Universitario Quiron, Pozuelo de Alarcon, Spain. [Holgado E] Medical Oncology Department, Hospital Universitario Ramon y Cajal, Madrid, Spain. [Manso L] Medical Oncology Department, Hospital Universitario 12 de Octubre, Madrid, Spain. [Morales S] Medical Oncology Department, Hospital Universitari Arnau Vilanova, Lleida, Spain. [Bermejo B] Medical Oncology Department, Hospital Clínico Universitario, Valencia, Spain. INCLIVA, Valencia, Spain. CIBERONC, Instituto Carlos III, Madrid, Spain. [Colomer R] Breast Cancer Clinical Research Unit – Clinical Research Program, CNIO - Spanish National Cancer Research Center, Melchor Fernandez Almagro, 3, 28029 Madrid, Spain. Medical Oncology Department, Hospital Universitario La Princesa, Madrid, Spain. Facultad de Medicina, Universidad Autónoma de Madrid, Madrid, Spain. [Cortes J] ION Institute of Oncology, Quironsalud Group – Madrid & Barcelona, Barcelona, Spain. Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain |
| dc.identifier.pmid | 33176887 |
| dc.identifier.wos | 000590839300002 |
| dc.relation.projectid | info:eu-repo/grantAgreement/ES/PE2013-2016/PI16%2F00354 |
| dc.relation.projectid | info:eu-repo/grantAgreement/ES/PE2013-2016/SAF2017-83732-R |
| dc.relation.projectid | info:eu-repo/grantAgreement/ES/PE2013-2016/PIE15%2F00068 |
| dc.relation.projectid | info:eu-repo/grantAgreement/ES/PE2013-2016/PI17%2F01865 |
| dc.rights.accessrights | info:eu-repo/semantics/openAccess |