| dc.contributor | Vall d'Hebron Barcelona Hospital Campus |
| dc.contributor.author | Hernando Cubero, Jorge |
| dc.contributor.author | Alonso, Lola |
| dc.contributor.author | Fasani, Roberta |
| dc.contributor.author | Jiménez Flores, José Antonio |
| dc.contributor.author | Elez Fernandez, Mª Elena |
| dc.contributor.author | Tabernero Caturla, Josep |
| dc.contributor.author | Nuciforo, Paolo Giovanni |
| dc.contributor.author | Capdevila Castillon, Jaume |
| dc.contributor.author | Landolfi, Stefania |
| dc.contributor.author | Comas Navarro, Raquel |
| dc.contributor.author | Ruiz Pace, Fiorella |
| dc.contributor.author | Serna, Garazi |
| dc.contributor.author | Dienstmann, Rodrigo |
| dc.date.accessioned | 2021-10-04T12:58:49Z |
| dc.date.available | 2021-10-04T12:58:49Z |
| dc.date.issued | 2020-10 |
| dc.identifier.citation | Serna G, Ruiz-Pace F, Hernando J, Alonso L, Fasani R, Landolfi S, et al. Fusobacterium nucleatum persistence and risk of recurrence after preoperative treatment in locally advanced rectal cancer. Ann Oncol. 2020 Oct;31(10):1366–75. |
| dc.identifier.issn | 0923-7534 |
| dc.identifier.uri | https://hdl.handle.net/11351/6378 |
| dc.description | Fusobacterium nucleatum; Locally advanced rectal cancer; Preoperative chemoradiotherapy |
| dc.description.abstract | Background
Accumulating evidence has identified Fusobacterium as an important pathogenic gut bacterium associated with colorectal cancer. Nevertheless, only limited data exist about the role of this bacterium in locally advanced rectal cancer (LARC). In this study, we quantified Fusobacterium nucleatum in untreated and post-neoadjuvant chemoradiotherapy (nCRT) samples from LARC patients and investigated its association with therapy response and survival.
Patients and methods
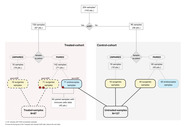
A total of 254 samples from 143 patients with rectal adenocarcinomas were analyzed for the presence and abundance of F. nucleatum using RNA in situ hybridization and digital image analysis. Assay accuracy was determined using infected cell lines and tumor samples with available quantitative PCR data. We studied the impact of F. nucleatum load on pathologic complete response and relapse-free survival. Treatment-induced changes were evaluated in paired pre- and post-nCRT samples (n = 71). Finally, tumor microenvironment changes during nCRT were assessed in paired samples (n = 45) by immune contexture analysis.
Results
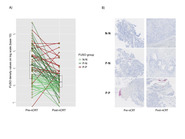
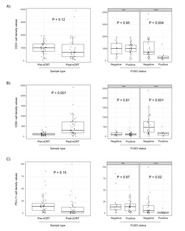
F. nucleatum tissue levels by RNA in situ hybridization strongly correlated with quantitative PCR (r = 0.804, P < 0.001). F. nucleatum abundance was higher in untreated [median, 7.4; 95% confidence interval (3.7–16.2)] compared with treated [median, 1.6; 95% confidence interval (1.3–2.4)] tumors (P <0.001) with 58% (73/126) and 26% (22/85) positive tumors, respectively (P < 0.001). Baseline F. nucleatum levels were not associated with pathologic complete response. F. nucleatum positivity after nCRT, but not baseline status, significantly increased risk of relapse [hazard ratio = 7.5, 95% confidence interval (3.0–19.0); P < 0.001]. Tumors that turned F. nucleatum-negative after nCRT had a strong increase in CD8+ T cells post-nCRT (P < 0.001), while those that persisted F. nucleatum-positive after nCRT lacked CD8+ T cells induction in post-nCRT samples compared with baseline (P = 0.69).
Conclusion
F. nucleatum persistence post-nCRT is associated with high relapse rates in LARC, potentially linked to suppression of immune cytotoxicity. |
| dc.language.iso | eng |
| dc.publisher | Elsevier |
| dc.relation.ispartofseries | Annals of Oncology;31(10) |
| dc.rights | Attribution-NonCommercial-NoDerivatives 4.0 International |
| dc.rights.uri | http://creativecommons.org/licenses/by-nc-nd/4.0/ |
| dc.source | Scientia |
| dc.subject | Bacteris gramnegatius |
| dc.subject | Recte - Càncer - Quimioteràpia |
| dc.subject | Recte - Càncer - Radioteràpia |
| dc.subject.mesh | Rectal Neoplasms |
| dc.subject.mesh | Fusobacterium nucleatum |
| dc.subject.mesh | Chemoradiotherapy |
| dc.title | Fusobacterium nucleatum persistence and risk of recurrence after preoperative treatment in locally advanced rectal cancer |
| dc.type | info:eu-repo/semantics/article |
| dc.identifier.doi | 10.1016/j.annonc.2020.06.003 |
| dc.subject.decs | neoplasias del recto |
| dc.subject.decs | Fusobacterium nucleatum |
| dc.subject.decs | quimiorradioterapia |
| dc.relation.publishversion | https://doi.org/10.1016/j.annonc.2020.06.003 |
| dc.type.version | info:eu-repo/semantics/publishedVersion |
| dc.audience | Professionals |
| dc.contributor.organismes | Institut Català de la Salut |
| dc.contributor.authoraffiliation | [Serna G, Alonso L, Fasani R, Jimenez J, Nuciforo P] Molecular Oncology Group, Vall d'Hebron Institute of Oncology (VHIO), Barcelona, Spain. [Ruiz-Pace F, Comas R, Dienstmann R] Oncology Data Science Group, Vall d'Hebron Institute of Oncology (VHIO), Barcelona, Spain. [Hernando J, Elez E, Capdevila J] Servei d’Oncologia Mèdica, Unitat de Tumors gastrointestinals i endocrins, Vall d'Hebron Hospital Universitari, Barcelona, Spain. Vall Hebron Institute of Oncology (VHIO), Barcelona, Spain. [Landolfi S] Servei d’Anatomia Patològica, Vall d'Hebron Hospital Universitari, Barcelona, Spain. [Tabernero J] Servei d’Oncologia Mèdica, Vall d'Hebron Hospital Universitari, Barcelona, Spain. Vall d'Hebron Institute of Oncology (VHIO), Barcelona, Spain. IOB-Quiron, UVic-UCC, Barcelona, Spain |
| dc.identifier.pmid | 32569727 |
| dc.identifier.wos | 000574680100001 |
| dc.rights.accessrights | info:eu-repo/semantics/openAccess |