| dc.contributor | Vall d'Hebron Barcelona Hospital Campus |
| dc.contributor.author | Jimenez-Fonseca, Paula |
| dc.contributor.author | Martinez-Torron, Alba |
| dc.contributor.author | Alsina Maqueda, Maria |
| dc.contributor.author | Custodio, Ana |
| dc.contributor.author | Serra, Olbia |
| dc.contributor.author | Carmona-Bayonas, Alberto |
| dc.date.accessioned | 2022-02-22T11:44:39Z |
| dc.date.available | 2022-02-22T11:44:39Z |
| dc.date.issued | 2021-01 |
| dc.identifier.citation | Jimenez-Fonseca P, Carmona-Bayonas A, Martinez-Torron A, Alsina M, Custodio A, Serra O, et al. External validity of clinical trials with diverse trastuzumab-based chemotherapy regimens in advanced gastroesophageal adenocarcinoma: data from the AGAMENON-SEOM registry. Ther Adv Med Oncol. 2021 Jan;13:1–13. |
| dc.identifier.issn | 1569-8041 |
| dc.identifier.uri | https://hdl.handle.net/11351/7066 |
| dc.description | Chemotherapy; Gastric cancer; Trastuzumab |
| dc.description.abstract | Background:
Trastuzumab combined with cisplatin and fluoropyrimidines, either capecitabine or 5-fluorouracile (XP/FP), is the standard first-line treatment for advanced, HER2-positive, gastric cancer patients based on the ToGA trial. Despite the lack of phase III trials, many clinicians administer trastuzumab with alternative regimens. One meta-analysis suggests that substituting cisplatin for oxaliplatin might lead to greater efficacy and less toxicity.
Methods:
594 patients with HER2-positive gastroesophageal adenocarcinoma were recruited from the AGAMENON-SEOM registry. The objective was to evaluate the external validity of clinical trials with chemotherapy and trastuzumab.
Results:
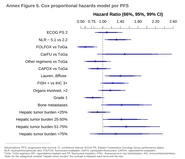
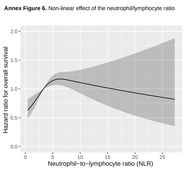
The regimens used in at least 5% of the patients were XP (27%), oxaliplatin and capecitabine (CAPOX) (26%), oxaliplatin and 5-fluorouracil (FOLFOX) (14%), FP (14%), triplet with anthracycline/docetaxel (7%), and carboplatin-FU (5%). Median exposure to trastuzumab was longer with FOLFOX (11.4 months, 95% CI, 9.1–21.0) versus ToGA regimens (7.5, 6.4–8.5), p < 0.001. Patients with HER2-IHC 3+ cancers had higher response rates than those with IHC 2+/FISH+, odds-ratio 1.97 (95% CI, 1.25–3.09). The results achieved with CAPOX–trastuzumab were comparable to those attained with ToGA regimens. FOLFOX–trastuzumab was superior to ToGA schemes in terms of overall survival (OS), with a greater magnitude of effect in IHC 2+/FISH+ tumors (HR 0.47, 0.24–0.92) compared with IHC 3+ (HR 0.69, 0.49–0.96), and in diffuse (HR 0.37, 0.20–0.69) versus intestinal-type tumors (HR 0.76, 0.54–1.06).
Conclusion:
We have updated the external validity of clinical trials with trastuzumab in first-line treatment of gastric cancer. Our data confirm the comparable outcomes of ToGA regimens and CAPOX–trastuzumab in clinical practice and point toward a possible benefit of FOLFOX–trastuzumab, contingent on the subtypes typically less sensitive to trastuzumab, to be confirmed in clinical trials. |
| dc.language.iso | eng |
| dc.publisher | SAGE Publications |
| dc.relation.ispartofseries | Therapeutic Advances in Medical Oncology;13 |
| dc.rights | Attribution-NonCommercial 4.0 International |
| dc.rights.uri | http://creativecommons.org/licenses/by-nc/4.0/ |
| dc.source | Scientia |
| dc.subject | Esòfag - Càncer - Tractament |
| dc.subject | Estómac - Càncer - Tractament |
| dc.subject | Quimioteràpia combinada |
| dc.subject.mesh | Esophageal Neoplasms |
| dc.subject.mesh | /drug therapy |
| dc.subject.mesh | Antineoplastic Combined Chemotherapy Protocols |
| dc.subject.mesh | /therapeutic use |
| dc.title | External validity of clinical trials with diverse trastuzumab-based chemotherapy regimens in advanced gastroesophageal adenocarcinoma: data from the AGAMENON-SEOM registry |
| dc.type | info:eu-repo/semantics/article |
| dc.identifier.doi | 10.1177/17588359211019672 |
| dc.subject.decs | neoplasias del esófago |
| dc.subject.decs | /farmacoterapia |
| dc.subject.decs | protocolos de quimioterapia antineoplásica combinada |
| dc.subject.decs | /uso terapéutico |
| dc.relation.publishversion | https://doi.org/10.1177/17588359211019672 |
| dc.type.version | info:eu-repo/semantics/publishedVersion |
| dc.audience | Professionals |
| dc.contributor.organismes | Institut Català de la Salut |
| dc.contributor.authoraffiliation | [Jimenez-Fonseca P] Medical Oncology Department, Hospital Universitario Central de Asturias, ISPA, Oviedo, Spain. [Carmona-Bayonas A] Medical Oncology Department, Hospital Universitario Morales Meseguer, 30007 Murcia, Spain. [Martinez-Torron A] Pharmacy Department, Hospital Universitario Marqués de Valdecilla, Santander, Spain. [Alsina M] Medical Oncology Department, Complejo Hospitalario de Navarra, Pamplona. Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain. [Custodio A] Medical Oncology Department, Hospital Universitario La Paz, CIBERONC CB16/12/00398, Madrid, Spain. [Serra O] Medical Oncology Department, Catalan Institute of Oncology, L’Hospitalet, Spain |
| dc.identifier.pmid | 34211587 |
| dc.identifier.wos | 000688566300001 |
| dc.rights.accessrights | info:eu-repo/semantics/openAccess |