| dc.contributor | Vall d'Hebron Barcelona Hospital Campus |
| dc.contributor.author | Swartzwelter, Benjamin J. |
| dc.contributor.author | Michelini, Sara |
| dc.contributor.author | Frauenlob, Tobias |
| dc.contributor.author | Barbero, Francesco |
| dc.contributor.author | Verde, Alessandro |
| dc.contributor.author | De Luca, Anna Chiara |
| dc.contributor.author | Franco Puntes, Victor |
| dc.date.accessioned | 2022-05-18T08:16:17Z |
| dc.date.available | 2022-05-18T08:16:17Z |
| dc.date.issued | 2021-11 |
| dc.identifier.citation | Swartzwelter BJ, Michelini S, Frauenlob T, Barbero F, Verde A, De Luca AC, et al. Innate Memory Reprogramming by Gold Nanoparticles Depends on the Microbial Agents That Induce Memory. Front Immunol. Nov 2021;12:751683. |
| dc.identifier.issn | 1664-3224 |
| dc.identifier.uri | https://hdl.handle.net/11351/7546 |
| dc.description | Innate immunity; Microbial agents; Nanoparticles |
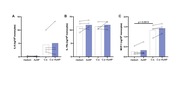
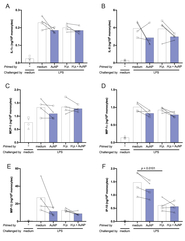
| dc.description.abstract | Innate immune memory, the ability of innate cells to react in a more protective way to secondary challenges, is induced by exposure to infectious and other exogeous and endogenous agents. Engineered nanoparticles are particulate exogenous agents that, as such, could trigger an inflammatory reaction in monocytes and macrophages and could therefore be also able to induce innate memory. Here, we have evaluated the capacity of engineered gold nanoparticles (AuNPs) to induce a memory response or to modulate the memory responses induced by microbial agents. Microbial agents used were in soluble vs. particulate form (MDP and the gram-positive bacteria Staphylococcus aureus; β-glucan and the β-glucan-producing fungi C. albicans), and as whole microrganisms that were either killed (S. aureus, C. albicans) or viable (the gram-negative bacteria Helicobacter pylori). The memory response was assessed in vitro, by exposing human primary monocytes from 2-7 individual donors to microbial agents with or without AuNPs (primary response), then resting them for 6 days to allow return to baseline, and eventually challenging them with LPS (secondary memory response). Primary and memory responses were tested as production of the innate/inflammatory cytokine TNFα and other inflammatory and anti-inflammatory factors. While inactive on the response induced by soluble microbial stimuli (muramyl dipeptide -MDP-, β-glucan), AuNPs partially reduced the primary response induced by whole microorganisms. AuNPs were also unable to directly induce a memory response but could modulate stimulus-induced memory in a circumscribed fashion, limited to some agents and some cytokines. Thus, the MDP-induced tolerance in terms of TNFα production was further exacerbated by co-priming with AuNPs, resulting in a less inflammatory memory response. Conversely, the H. pylori-induced tolerance was downregulated by AuNPs only relative to the anti-inflammatory cytokine IL-10, which would lead to an overall more inflammatory memory response. These effects of AuNPs may depend on a differential interaction/association between the reactive particle surfaces and the microbial components and agents, which may lead to a change in the exposure profiles. As a general observation, however, the donor-to-donor variability in memory response profiles and reactivity to AuNPs was substantial, suggesting that innate memory depends on the individual history of exposures. |
| dc.language.iso | eng |
| dc.publisher | Frontiers Media |
| dc.relation.ispartofseries | Frontiers in Immunology;12 |
| dc.rights | Attribution 4.0 International |
| dc.rights.uri | http://creativecommons.org/licenses/by/4.0/ |
| dc.source | Scientia |
| dc.subject | Nanopartícules |
| dc.subject | Monòcits - Immunologia |
| dc.subject | Memòria - Efecte dels medicaments |
| dc.subject.mesh | Metal Nanoparticles |
| dc.subject.mesh | /administration & dosage |
| dc.subject.mesh | Immunologic Memory |
| dc.subject.mesh | Monocytes |
| dc.title | Innate Memory Reprogramming by Gold Nanoparticles Depends on the Microbial Agents That Induce Memory |
| dc.type | info:eu-repo/semantics/article |
| dc.identifier.doi | 10.3389/fimmu.2021.751683 |
| dc.subject.decs | nanopartículas metálicas |
| dc.subject.decs | /administración & dosificación |
| dc.subject.decs | memoria inmunológica |
| dc.subject.decs | monocitos |
| dc.relation.publishversion | https://doi.org/10.3389/fimmu.2021.751683 |
| dc.type.version | info:eu-repo/semantics/publishedVersion |
| dc.audience | Professionals |
| dc.contributor.organismes | Institut Català de la Salut |
| dc.contributor.authoraffiliation | [Swartzwelter BJ] Institute of Biochemistry and Cell Biology (IBBC), National Research Council (CNR), Napoli, Italy. Department Biosciences, Paris Lodron University of Salzburg (PLUS), Salzburg, Austria. [Michelini S, Frauenlob T] Department Biosciences, Paris Lodron University of Salzburg (PLUS), Salzburg, Austria. [Barbero F] Institut Català de Nanociència i Nanotecnologia (ICN2), Consejo Superior de Investigaciones Científicas (CSIC) and The Barcelona Institute of Science and Technology (BIST), Barcelona, Spain. [Verde A, De Luca AC] Institute of Biochemistry and Cell Biology (IBBC), National Research Council (CNR), Napoli, Italy. [Puntes V] Institut Català de Nanociència i Nanotecnologia (ICN2), Consejo Superior de Investigaciones Científicas (CSIC) and The Barcelona Institute of Science and Technology (BIST), Barcelona, Spain. Vall d’Hebron Institut de Recerca (VHIR), Barcelona, Spain. Institució Catalana de Recerca I Estudis Avançats (ICREA), Barcelona, Spain |
| dc.identifier.pmid | 34804037 |
| dc.identifier.wos | 000720305300001 |
| dc.rights.accessrights | info:eu-repo/semantics/openAccess |