| dc.contributor | Vall d'Hebron Barcelona Hospital Campus |
| dc.contributor.author | Harbeck, Nadia |
| dc.contributor.author | Rastogi, P. |
| dc.contributor.author | Martin, M. |
| dc.contributor.author | Tolaney, Sara M |
| dc.contributor.author | Shao, Zhi Min |
| dc.contributor.author | Cortés Castan, Javier |
| dc.contributor.author | Fasching, Peter A. |
| dc.date.accessioned | 2022-06-13T09:42:57Z |
| dc.date.available | 2022-06-13T09:42:57Z |
| dc.date.issued | 2021-12 |
| dc.identifier.citation | Harbeck N, Rastogi P, Martin M, Tolaney SM, Shao ZM, Fasching PA, et al. Adjuvant Abemaciclib Combined With Endocrine Therapy for High-Risk Early Breast Cancer: Updated Efficacy and Ki-67 Analysis From the monarchE Study. Ann Oncol. 2021 Dec;32(12):1571–81. |
| dc.identifier.issn | 0923-7534 |
| dc.identifier.uri | https://hdl.handle.net/11351/7662 |
| dc.description | Abemaciclib; Adjuvant; Early breast cancer |
| dc.description.abstract | Background
Adjuvant abemaciclib combined with endocrine therapy (ET) previously demonstrated clinically meaningful improvement in invasive disease-free survival (IDFS) and distant relapse-free survival (DRFS) in hormone receptor-positive, human epidermal growth factor receptor 2-negative, node-positive, high-risk early breast cancer at the second interim analysis, however follow-up was limited. Here, we present results of the prespecified primary outcome analysis and an additional follow-up analysis.
Patients and methods
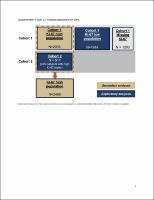
This global, phase III, open-label trial randomized (1 : 1) 5637 patients to adjuvant ET for ≥5 years ± abemaciclib for 2 years. Cohort 1 enrolled patients with ≥4 positive axillary lymph nodes (ALNs), or 1-3 positive ALNs and either grade 3 disease or tumor ≥5 cm. Cohort 2 enrolled patients with 1-3 positive ALNs and centrally determined high Ki-67 index (≥20%). The primary endpoint was IDFS in the intent-to-treat population (cohorts 1 and 2). Secondary endpoints were IDFS in patients with high Ki-67, DRFS, overall survival, and safety.
Results
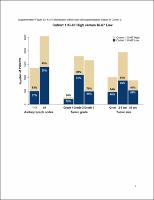
At the primary outcome analysis, with 19 months median follow-up time, abemaciclib + ET resulted in a 29% reduction in the risk of developing an IDFS event [hazard ratio (HR) = 0.71, 95% confidence interval (CI) 0.58-0.87; nominal P = 0.0009]. At the additional follow-up analysis, with 27 months median follow-up and 90% of patients off treatment, IDFS (HR = 0.70, 95% CI 0.59-0.82; nominal P < 0.0001) and DRFS (HR = 0.69, 95% CI 0.57-0.83; nominal P < 0.0001) benefit was maintained. The absolute improvements in 3-year IDFS and DRFS rates were 5.4% and 4.2%, respectively. Whereas Ki-67 index was prognostic, abemaciclib benefit was consistent regardless of Ki-67 index. Safety data were consistent with the known abemaciclib risk profile.
Conclusion
Abemaciclib + ET significantly improved IDFS in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative, node-positive, high-risk early breast cancer, with an acceptable safety profile. Ki-67 index was prognostic, but abemaciclib benefit was observed regardless of Ki-67 index. Overall, the robust treatment benefit of abemaciclib extended beyond the 2-year treatment period. |
| dc.language.iso | eng |
| dc.publisher | Elsevier |
| dc.relation.ispartofseries | Annals of Oncology;32(12) |
| dc.rights | Attribution-NonCommercial-NoDerivatives 4.0 International |
| dc.rights.uri | http://creativecommons.org/licenses/by-nc-nd/4.0/ |
| dc.source | Scientia |
| dc.subject | Mama - Càncer - Tractament |
| dc.subject | Quimioteràpia combinada |
| dc.subject.mesh | Breast Neoplasms |
| dc.subject.mesh | /drug therapy |
| dc.subject.mesh | Antineoplastic Combined Chemotherapy Protocols |
| dc.subject.mesh | /therapeutic use |
| dc.title | Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study |
| dc.type | info:eu-repo/semantics/article |
| dc.identifier.doi | 10.1016/j.annonc.2021.09.015 |
| dc.subject.decs | neoplasias de la mama |
| dc.subject.decs | /farmacoterapia |
| dc.subject.decs | protocolos de quimioterapia antineoplásica combinada |
| dc.subject.decs | /uso terapéutico |
| dc.relation.publishversion | https://doi.org/10.1016/j.annonc.2021.09.015 |
| dc.type.version | info:eu-repo/semantics/publishedVersion |
| dc.audience | Professionals |
| dc.contributor.organismes | Institut Català de la Salut |
| dc.contributor.authoraffiliation | [Harbeck N] Breast Center, Department of OB & GYN and CCC Munich, LMU University Hospital, Munich, Germany. [Rastogi P] University of Pittsburgh/UPMC, NSABP Foundation, Pittsburgh, USA. [Martin M] Hospital General Universitario Gregorio Marañon, Universidad Complutense, CIBERONC, GEICAM, Madrid, Spain. [Tolaney SM] Dana-Farber Cancer Institute, Boston, USA. [Shao ZM] Fudan University Shanghai Cancer Center, Shanghai, China. [Fasching PA] University Hospital Erlangen, Department of Gynecology and Obstetrics, Comprehensive Cancer Center Erlangen-EMN, Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany. [Cortés J] International Breast Cancer Center (IBCC), Madrid & Barcelona. Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain. Universidad Europea de Madrid, Faculty of Biomedical and Health Sciences, Department of Medicine, Madrid, Spain |
| dc.identifier.pmid | 34656740 |
| dc.identifier.wos | 000721610600014 |
| dc.rights.accessrights | info:eu-repo/semantics/openAccess |