| dc.contributor | Vall d'Hebron Barcelona Hospital Campus |
| dc.contributor.author | Bordonaro, R. |
| dc.contributor.author | Calvo, A. |
| dc.contributor.author | Auriemma, A. |
| dc.contributor.author | Rubovszky, G. |
| dc.contributor.author | Saunders, M. P. |
| dc.contributor.author | Argilés Martinez, Guillem |
| dc.contributor.author | Tabernero, Josep |
| dc.contributor.author | Hollebecque, Antoine |
| dc.date.accessioned | 2022-04-22T13:36:20Z |
| dc.date.available | 2022-04-22T13:36:20Z |
| dc.date.issued | 2021-10 |
| dc.identifier.citation | Bordonaro R, Calvo A, Auriemma A, Hollebecque A, Rubovszky G, Saunders MP, et al. Trifluridine/tipiracil in combination with oxaliplatin and either bevacizumab or nivolumab in metastatic colorectal cancer: a dose-expansion, phase I study. ESMO Open. 2021 Oct 1;6(5):100270. |
| dc.identifier.issn | 2059-7029 |
| dc.identifier.uri | https://hdl.handle.net/11351/7384 |
| dc.description | Metastatic colorectal cancer; Oxaliplatin; Trifluridine/tipiracil |
| dc.description.abstract | Background
In preclinical studies trifluridine/tipiracil (FTD/TPI) plus oxaliplatin (Industriestrasse, Holzkirchen, Germany) sensitised microsatellite stable (MSS) metastatic colorectal cancer (mCRC) to anti-programmed cell death protein-1; the addition of oxaliplatin or bevacizumab (F Hoffmann- la ROCHE AG, Kaiseraugst, Switzerland) enhanced the antitumour effects of FTD/TPI. This study aimed to investigate the safety and efficacy of FTD/TPI plus oxaliplatin and either bevacizumab or nivolumab (Uxbridge business Park, Uxbridge, United Kingdom) in patients with mCRC who had progressed after at least one prior line of treatment.
Patients and methods
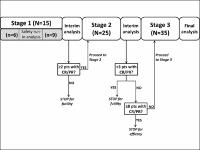
In 14-day cycles, patients received FTD/TPI 35 mg/m2 (twice daily, days 1-5) plus oxaliplatin 85 mg/m2 (day 1), and, on day 1, either bevacizumab 5 mg/kg (cohort A) or nivolumab 3 mg/kg (cohort B). Patients in Cohort B had confirmed MSS status.
Results
In total, 54 patients were enrolled: 37 in cohort A and 17 in cohort B. Recruitment in cohort B was stopped early due to the low response rate (RR) observed at interim analyses of efficacy. The most common adverse events (AEs) in cohort A were neutropenia/decreased neutrophils (75.7%), nausea (59.5%), vomiting (40.5%), diarrhoea (37.8%), peripheral sensory neuropathy (37.8%), fatigue (35.1%) and decreased appetite (35.1%). In cohort B, the most common AEs were neutropenia/decreased neutrophils (70.6%), diarrhoea (58.8%), nausea (47.1%), vomiting (47.1%), fatigue (47.1%), asthenia (41.2%), paraesthesia (41.2%), thrombocytopenia/decreased platelets (35.3%) and decreased appetite (35.3%). Confirmed objective RR was 17.1% in cohort A and 7.1% in cohort B; the corresponding values for median progression-free survival in the two cohorts were 6.3 and 6.0 months.
Conclusion
FTD/TPI plus oxaliplatin and bevacizumab or nivolumab had an acceptable safety profile and demonstrated antitumour activity in previously treated patients with mCRC. |
| dc.language.iso | eng |
| dc.publisher | Elsevier |
| dc.relation.ispartofseries | ESMO Open;6(5) |
| dc.rights | Attribution-NonCommercial-NoDerivatives 4.0 International |
| dc.rights.uri | http://creativecommons.org/licenses/by-nc-nd/4.0/ |
| dc.source | Scientia |
| dc.subject | Còlon - Càncer - Tractament |
| dc.subject | Recte - Càncer - Tractament |
| dc.subject | Quimioteràpia combinada |
| dc.subject.mesh | Colorectal Neoplasms |
| dc.subject.mesh | /drug therapy |
| dc.subject.mesh | Antineoplastic Combined Chemotherapy Protocols |
| dc.subject.mesh | /adverse effects |
| dc.title | Trifluridine/tipiracil in combination with oxaliplatin and either bevacizumab or nivolumab in metastatic colorectal cancer: a dose-expansion, phase I study |
| dc.type | info:eu-repo/semantics/article |
| dc.identifier.doi | 10.1016/j.esmoop.2021.100270 |
| dc.subject.decs | neoplasias colorrectales |
| dc.subject.decs | /farmacoterapia |
| dc.subject.decs | protocolos de quimioterapia antineoplásica combinada |
| dc.subject.decs | /efectos adversos |
| dc.relation.publishversion | https://doi.org/10.1016/j.esmoop.2021.100270 |
| dc.type.version | info:eu-repo/semantics/publishedVersion |
| dc.audience | Professionals |
| dc.contributor.organismes | Institut Català de la Salut |
| dc.contributor.authoraffiliation | [Bordonaro R] Azienda Ospedaliera ARNAS Garibaldi, Catania, Italy. [Calvo A] Gregorio Marañon University General Hospital, Madrid, Spain. [Auriemma A] Azienda Ospedaliera Universitaria Integrat, University of Verona, Verona, Italy. [Hollebecque A] Drug Development Department, Gustave Roussy Cancer Campus, Villejuif, France. [Rubovszky G] Department of Medical Oncology and Clinical Pharmacology, National Institute of Oncology Hungary, Budapest, Hungary. [Saunders MP] Christie NHS Foundation Trust, Manchester, UK. [Argilés G] Vall d'Hebron Hospital Universitari, Barcelona, Spain. Vall d'Hebron Institute of Oncology (VHIO), Barcelona, Spain. [Tabernero J] Vall d'Hebron Hospital Universitari, Barcelona, Spain. Vall d'Hebron Institute of Oncology (VHIO), Barcelona, Spain. UVic-UCC, IOB-Quiron, Barcelona, Spain |
| dc.identifier.pmid | 34547581 |
| dc.identifier.wos | 000704803800020 |
| dc.rights.accessrights | info:eu-repo/semantics/openAccess |

 Private area
Private area Contact Us
Contact Us