| dc.contributor | Vall d'Hebron Barcelona Hospital Campus |
| dc.contributor.author | Tolaney, Sara M |
| dc.contributor.author | Loirat, Delphine |
| dc.contributor.author | Antunes de Melo Oliveira, Ana Mafalda |
| dc.contributor.author | Kalinsky, K. |
| dc.contributor.author | Bardia, Aditya |
| dc.contributor.author | Punie, Kevin |
| dc.date.accessioned | 2022-06-13T10:42:41Z |
| dc.date.available | 2022-06-13T10:42:41Z |
| dc.date.issued | 2021-09 |
| dc.identifier.citation | Bardia A, Tolaney SM, Punie K, Loirat D, Oliveira M, Kalinsky K, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021 Sep;32(9):1148–56. |
| dc.identifier.issn | 0923-7534 |
| dc.identifier.uri | https://hdl.handle.net/11351/7666 |
| dc.description | BRCA; Triple-negative breast cancer; Trophoblast cell-surface antigen 2 |
| dc.description.abstract | Background
The pivotal phase III ASCENT trial demonstrated improved survival outcomes associated with sacituzumab govitecan (SG), an anti-trophoblast cell-surface antigen 2 (anti-Trop-2) antibody-drug conjugate linked with the topoisomerase-inhibitor SN-38, over single-agent chemotherapy treatment of physician’s choice (TPC) in previously treated metastatic triple-negative breast cancer (mTNBC). This prespecified, exploratory biomarker analysis from the ASCENT trial evaluates the association between tumor Trop-2 expression and germline BRCA1/2 mutation status with clinical outcomes.
Patients and methods
Patients with mTNBC refractory to or progressing after two or more prior chemotherapies, with one or more in the metastatic setting, were randomized to receive SG (10 mg/kg intravenously days 1 and 8, every 21 days) or TPC (capecitabine, eribulin, vinorelbine, or gemcitabine) until disease progression/unacceptable toxicity. Biopsy or surgical specimens were collected at study entry to determine Trop-2 expression level using a validated immunohistochemistry assay and histochemical scoring. Germline BRCA1/2 mutation status was collected at baseline.
Results
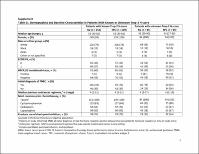
Of 468 assessable patients, 290 had Trop-2 expression data [64% (n = 151 SG) versus 60% (n = 139 TPC)] and 292 had known BRCA1/2 mutation status [63% (n = 149 SG) versus 61% (n = 143 TPC)]. Median progression-free survival in SG- versus TPC-treated patients was 6.9, 5.6, and 2.7 months versus 2.5, 2.2, and 1.6 months for high, medium, and low Trop-2 expression, respectively. Median overall survival (14.2, 14.9, and 9.3 months versus 6.9, 6.9, and 7.6 months) and objective response rates (44%, 38%, and 22% versus 1%, 11%, and 6%) were numerically higher with SG versus TPC in patients with high, medium, and low Trop-2 expression, respectively. Efficacy outcomes were numerically higher with SG versus TPC in patients with and without germline BRCA1/2 mutations.
Conclusions
SG benefits patients with previously treated mTNBC expressing high/medium Trop-2 compared with standard-of-care chemotherapy and regardless of germline BRCA1/2 mutation status. The small number of patients with low Trop-2 expression precludes definitive conclusions on the benefit of SG in this subgroup. |
| dc.language.iso | eng |
| dc.publisher | Elsevier |
| dc.relation.ispartofseries | Annals of Oncology;32(9) |
| dc.rights | Attribution 4.0 International |
| dc.rights.uri | http://creativecommons.org/licenses/by/4.0/ |
| dc.source | Scientia |
| dc.subject | Mama - Càncer - Tractament |
| dc.subject | Immunoglobulines - Ús terapèutic |
| dc.subject | Marcadors bioquímics - Anàlisi |
| dc.subject.mesh | Breast Neoplasms |
| dc.subject.mesh | /drug therapy |
| dc.subject.mesh | Immunoconjugates |
| dc.subject.mesh | /therapeutic use |
| dc.title | Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer |
| dc.type | info:eu-repo/semantics/article |
| dc.identifier.doi | 10.1016/j.annonc.2021.06.002 |
| dc.subject.decs | neoplasias de la mama |
| dc.subject.decs | /farmacoterapia |
| dc.subject.decs | inmunoconjugados |
| dc.subject.decs | /uso terapéutico |
| dc.relation.publishversion | https://doi.org/10.1016/j.annonc.2021.06.002 |
| dc.type.version | info:eu-repo/semantics/publishedVersion |
| dc.audience | Professionals |
| dc.contributor.organismes | Institut Català de la Salut |
| dc.contributor.authoraffiliation | [Bardia A] Massachusetts General Hospital, Harvard Medical School, Boston, USA. [Tolaney SM] Medical Oncology, Dana-Farber Cancer Institute, Boston, USA. [Punie K] Department of General Medical Oncology and Multidisciplinary Breast Centre, Leuven Cancer Institute, University Hospitals Leuven, Leuven, Belgium. [Loirat D] Medical Oncology Department and D3i, Institut Curie, Paris, France. [Oliveira M] Vall d’Hebron Hospital Universitari, Barcelona, Spain. [Kalinsky K] Columbia University Irving Medical Center, New York, USA. Winship Cancer Institute, Emory University, Atlanta, USA |
| dc.identifier.pmid | 34116144 |
| dc.identifier.wos | 000687824100010 |
| dc.rights.accessrights | info:eu-repo/semantics/openAccess |

 Private area
Private area Contact Us
Contact Us