| dc.contributor | Vall d'Hebron Barcelona Hospital Campus |
| dc.contributor.author | Jimenez-Fonseca, Paula |
| dc.contributor.author | Martínez de Castro, Eva |
| dc.contributor.author | Custodio, Ana |
| dc.contributor.author | Pericay, Carles |
| dc.contributor.author | Hernández, Raquel |
| dc.contributor.author | Diez Garcia, Marc |
| dc.contributor.author | Carmona-Bayonas, Alberto |
| dc.date.accessioned | 2022-06-28T10:25:07Z |
| dc.date.available | 2022-06-28T10:25:07Z |
| dc.date.issued | 2021-03 |
| dc.identifier.citation | Jimenez-Fonseca P, Carmona-Bayonas A, Martínez de Castro E, Custodio A, Pericay Pijaume C, Hernandez R, et al. External validity of docetaxel triplet trials in advanced gastric cancer: are there patients who still benefit? Gastric Cancer. 2021 Mar;24:445–56. |
| dc.identifier.issn | 1436-3305 |
| dc.identifier.uri | https://hdl.handle.net/11351/7737 |
| dc.description | Bayesian model; Docetaxel; Gastric cancer |
| dc.description.abstract | Background
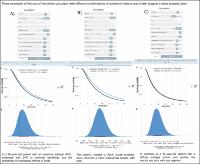
The purpose of our study was to develop an online calculator to estimate the effect of docetaxel triplets (DPF) in first line of advanced gastric cancer (AGC), and to assess the external validity of docetaxel trials in individual patients.
Methods
The study includes patients with HER2(-) AGC treated with platin and fluoropyrimidine (PF) or with DPF in first line. Treatment effect and interactions were assessed using Bayesian accelerated failure time models.
Result
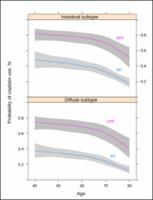
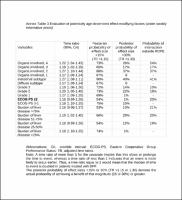
The series comprises 1376 patients; 238 treated with DPF and 1138 with PF between 2008 and 2019. DPF was associated with increased progression-free survival (PFS) and overall survival (OS) with time ratio (TR) 1.27 (95% credible interval [CrI], 1.15–1.40), and TR 1.19 (95% CrI, 1.09–1.27), respectively. Serious adverse events were more common with DPF, particularly hematological effects (32% vs 22%). Younger participants received greater DPF dose density without achieving greater disease control, while severe toxicity was likewise higher. DPF yielded superior OS in Lauren intestinal (TR 1.27, 95% CrI, 1.08–1.11) vs diffuse subtype (TR 1.17, 95% CrI, 1.09–1.24) and the probability of increasing OS > 15% was 90% vs 67% in each subtype, respectively. The effect dwindles over time, which can be attributed to pathological changes and clinical practice changes.
Conclusion
Our study confirms the effect of DPF is highly dependent on several clinical–pathological variables, with discreet and gradually declining benefit over platinum doublets in later years, at the expense of increased toxicity. These results may help to underpin the idea that external validity of AGC trials should be revised regularly. |
| dc.language.iso | eng |
| dc.publisher | Springer |
| dc.relation.ispartofseries | Gastric Cancer;24 |
| dc.rights | Attribution 4.0 International |
| dc.rights.uri | http://creativecommons.org/licenses/by/4.0/ |
| dc.source | Scientia |
| dc.subject | Estómac - Càncer - Tractament |
| dc.subject | Estadística bayesiana |
| dc.subject | Anàlisi de supervivència (Biometria) |
| dc.subject.mesh | Stomach Neoplasms |
| dc.subject.mesh | /drug therapy |
| dc.subject.mesh | Progression-Free Survival |
| dc.subject.mesh | Bayes Theorem |
| dc.title | External validity of docetaxel triplet trials in advanced gastric cancer: are there patients who still benefit? |
| dc.type | info:eu-repo/semantics/article |
| dc.identifier.doi | 10.1007/s10120-020-01116-x |
| dc.subject.decs | neoplasias gástricas |
| dc.subject.decs | /farmacoterapia |
| dc.subject.decs | supervivencia libre de progresión |
| dc.subject.decs | teorema de Bayes |
| dc.relation.publishversion | https://doi.org/10.1007/s10120-020-01116-x |
| dc.type.version | info:eu-repo/semantics/publishedVersion |
| dc.audience | Professionals |
| dc.contributor.organismes | Institut Català de la Salut |
| dc.contributor.authoraffiliation | [Jimenez-Fonseca P] Medical Oncology Department, Hospital Universitario Central de Asturias, ISPA, Oviedo, Spain. [Carmona-Bayonas A] Hematology and Medical Oncology Department, Hospital Universitario Morales Meseguer, University of Murcia, Murcia, Spain. Fundación Séneca, Murcia, Spain. [Martínez de Castro E] Medical Oncology Department, Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain. [Custodio A] Medical Oncology Department, Hospital Universitario La Paz, Madrid, Spain. [Pericay Pijaume C] Medical Oncology Department, Hospital Universitario Parc Tauli, Sabadell, Spain. [Hernandez R] Medical Oncology Department, Hospital Universitario de Canarias, Tenerife, Spain. [Diez M] Servei d’Oncologia Mèdica, Vall d’Hebron Hospital Universitari, Barcelona, Spain |
| dc.identifier.pmid | 32970266 |
| dc.identifier.wos | 000572609700002 |
| dc.rights.accessrights | info:eu-repo/semantics/openAccess |

 Private area
Private area Contact Us
Contact Us